Abstract
Background R/R T and NK-cell lymphoma has a dismal prognosis owing to its aggressiveness and limited therapeutic approaches. ATG-010 (selinexor), an exportin 1 (XPO1) protein inhibitor, when combined with GemOx regimen, has demonstrated preliminary efficacy in Chinese patients (pts) with R/R T and NK-cell lymphoma in the Phase Ib TOUCH study. This report is an update of this trial with cumulative data of approximately 22 months.
Method The study was designed to evaluate safety and efficacy of ATG-010 (60mg Days 1, 8) plus standard dose of chemotherapy (GemOx or ICE regimen as per investigator's choice) every 3 weeks (2-6 cycles), followed by ATG-010 maintenance (60 mg weekly) in pts with R/R T and NK-cell lymphoma in China, including subtypes of PTCL-NOS, ENKTL, AITL, ALCL, PTCL-TFH and FTCL. Considering the positive signal observed in GemOx arm, the study was amended to expand enrollment in GemOx arm to a maximum of 46 pts. Meanwhile, recruitment of ICE arm was stopped due to slow accrual and poor tolerability. Eligibility criteria, study treatment and study endpoints remained the same as in the original protocol. This study was registered at ClinicalTrials.gov (NCT04425070).
Results From Aug 18, 2020 to June 10, 2022, 39 pts (35 in GemOx arm, 4 in ICE arm) were enrolled and received at least one dose of study treatment. Results of the GemOx arm are updated in this report. At study entry, median age was 55 years (range 25-78); 29 (82.9%) pts had stage III/IV disease. Disease subtypes included 15 (42.9%) PTCL-NOS, 10 (28.6%) ENKTL, 9 (25.7%) AITL and 1 (2.9%) ALK (-) ALCL. Seven (20%) pts with PTCL had an IPI score ≥3, and 4 (11.4%) ENKTL pts had a PINK-E score ≥3. Median number of prior regimens was 3 (range 1-10); 33 (94.3%) pts were refractory to last-line therapy. Twenty (57.1%) pts had prior gemcitabine exposure, 20 (57.1%) with HDACi (chidamide) exposure, and 11 (31.4%) with prior anti-PD-1/PD-L1 therapy. Median time from last therapy to study entry was 1.4 months (range 0.7-38.3). Median duration of study treatment was 12.1 weeks (range 2.7-49.7). Relative dose intensity of ATG-010 was 80.6% when combined with GemOx, and 90.6% when as monotherapy in maintenance. Twenty-one (60%) pts received a systemic anti-cancer therapy post study treatment.
All pts experienced at least one treatment-emergent adverse event (TEAE), 34 (97.1%) pts with at least one Grade≥3 TEAE, and 16 (45.7%) pts with at least one serious TEAE. One pt discontinued treatment due to AE (neutropenia), and one G5 AE (death NOS) occurred which was considered not related to treatment but most likely due to disease progression. The most common TEAEs were hematological toxicities. Grade≥3 hematological TEAEs included thrombocytopenia (80%), neutropenia (74.3%), and anemia (42.9%). Grade≥3 non-hematological TEAEs (≥5%) were diarrhea (11.4%), pyrexia (8.6%), and hyponatremia (5.7%). The most common serious TEAE was thrombocytopenia (28.6%). The majority of toxicities were manageable by dose modification and supportive care.
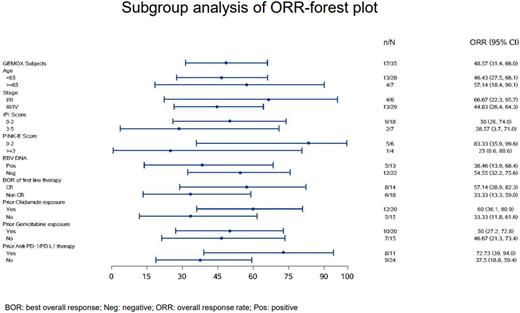
Amongst 35 efficacy-evaluable GemOx pts, ORR was 48.6% (17/35); CR rate was 22.9% (8/35). ORR of PTCL-NOS, ENKTL, AITL, ALCL pts were 53.3% (8/15), 60% (6/10), 22.2% (2/9), and 100% (1/1); CR rates were 26.7%, 20%, 11.1%, and 100%, respectively. ORR was similar between pts with (50%, 10/20) and without (46.7%, 7/15) prior gemcitabine exposure. Higher ORR was observed in pts with (60%, 12/20) prior chidamide exposure when compared with pts without (33.3%, 5/15) chidamide exposure, and in pts with (72.7%, 8/11) prior anti-PD-1/PD-L1 therapy when compared with pts without (37.5%, 9/24) anti-PD1/PD-L1 exposure. At a median follow-up of 13.0 months, median PFS, DOR and OS of the 35 GemOx pts were 2.9 (95% CI 1.5-4.7), 3.3 (95% CI 1.6-8.1) months, and not reached (95% CI 11.5-NE), respectively. Median PFS for PTCL-NOS and ENKTL pts were 4.4 (95% CI 1.5-9.6) and 4.7 (95% CI 1.2-NE) months, respectively. Median OS for PTCL-NOS, ENKTL and AITL were not reached (95% CI 5.6-NE), 14.1 (95% CI 8.3-NE) months, and not reached (95% CI 2.2-NE), respectively.
Conclusions ATG-010 plus GemOx regimen demonstrated a manageable safety profile and favorable efficacy in pts with R/R T and NK-cell lymphoma, especially PTCL-NOS and ENKTL, potentially offering a new therapeutic option for this heavily pretreated patient population.
Disclosures
Lou:Antengene Therapeutics Ltd.: Current Employment. Fei:Antengene Therapeutics Ltd.: Current Employment. Wang:Antengene Therapeutics Ltd.: Ended employment in the past 24 months. Lynch:Antengene Therapeutics Ltd.: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal